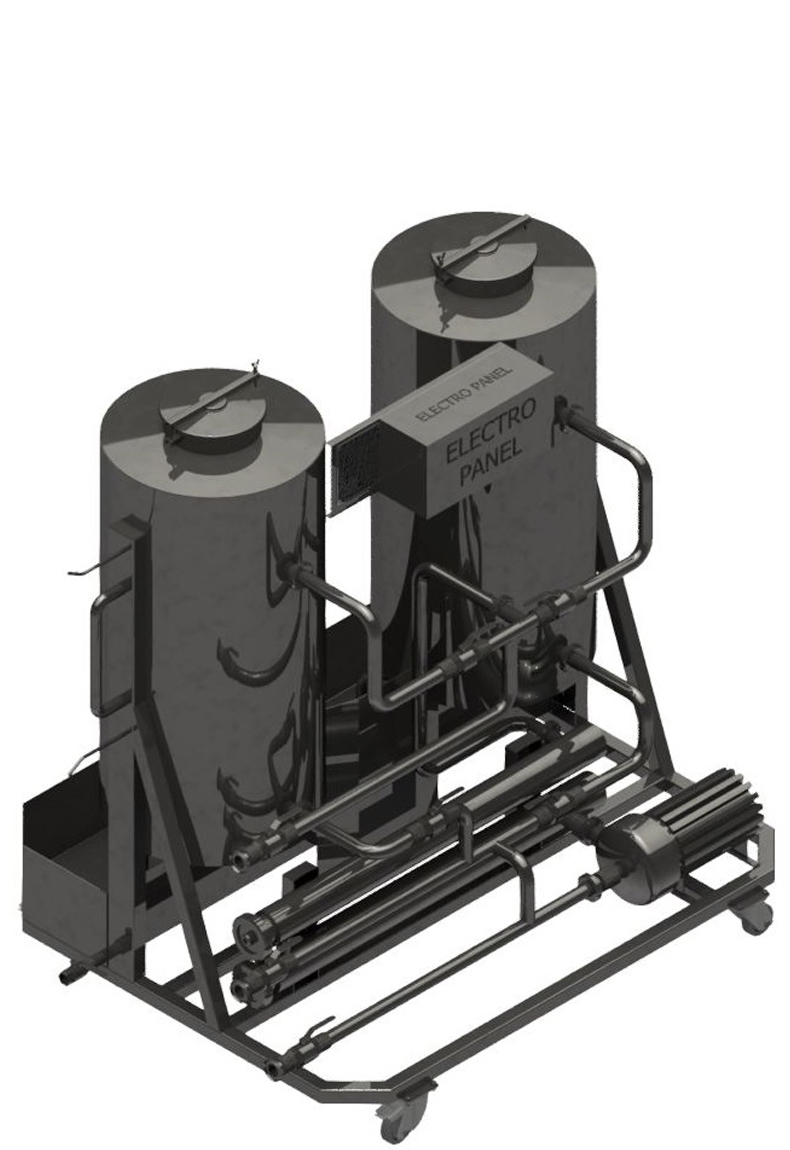
CIP cleaning
and sanitation
- Full stainless steel CIP unit
- Mobile frame
- El. heating with very fast performance
- Neutralization vat for waste solution
- Made in Czech Republic



About CIP
Clean-in-place (CIP) is a method of cleaning the interior surfaces of pipes, vessels, process equipment, filters and associated fittings, without disassembly.
Up to the 1950s, closed systems were disassembled and cleaned manually. The advent of CIP was a boon to industries that needed frequent internal cleaning of their processes. Industries that rely heavily on CIP are those requiring high levels of hygiene, and include: dairy, beverage, brewing, processed foods, pharmaceutical, and cosmetics.
The benefit to industries that use CIP is that the cleaning is faster, less labor-intensive and more repeatable, and poses less of a chemical exposure risk to people. CIP started as a manual practice involving a balance tank, centrifugal pump, and connection to the system being cleaned. Since the 1950s, CIP has evolved to include fully automated systems with programmable logic controllers, multiple balance tanks, sensors, valves, heat exchangers, data acquisition and specially designed spray nozzle systems. Simple, manually operated CIP systems can still be found in use today.
CIP is commonly used for cleaning bioreactors, fermenters, mix vessels, and other equipment used in biotech manufacturing, pharmaceutical manufacturing and food and beverage manufacturing. CIP is performed to remove or obliterate previous Cell Culture batch components. It is used to remove in-process residues, control bioburden, and reduce endotoxin levels within processing equipment and systems. Residue removal is accomplished during CIP with a combination of heat, chemical action, and turbulent flow.
The code of Europe regulations states “Equipment and utensils shall be cleaned, maintained, and sanitized at appropriate intervals to prevent malfunctions or contamination that would alter the safety, identity, strength, quality or purity of the drug product beyond the official or other established requirements.
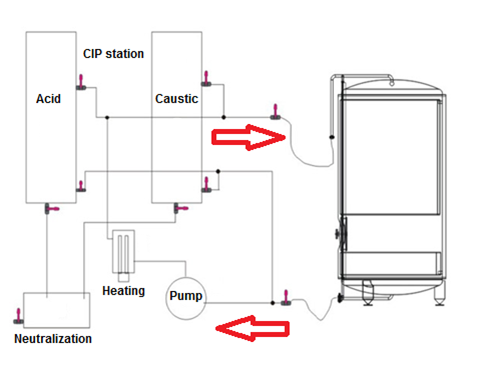
Repeatable, reliable, and effective cleaning is of the utmost importance in a manufacturing facility. Cleaning procedures are validated to demonstrate that they are effective, reproducible, and under control. In order to adequately clean processing equipment, the equipment must be designed with smooth stainless steel surfaces and interconnecting piping that has cleanable joints. The chemical properties of the cleaning agents must properly interact with the chemical and physical properties of the residues being removed. A typical CIP cycle consists of many steps which often include (in order):
- Pre-rinse with WFI (water for injection) or PW (purified water) which is performed to wet the interior surface of the tank and remove residue. It also provides a non-chemical pressure test of the CIP flow path.
- Caustic solution single pass flush through the vessel to drain. Caustic is the main cleaning solution.
- Caustic solution re-circulation through the vessel.
- Intermediate WFI or PW rinse
- Acid solution wash – used to remove mineral precipitates and protein residues
- Final rinse with WFI or PW – rinses to flush out residual cleaning agents.
- Final air blow – used to remove moisture remaining after CIP cycle.
References
Our quality work is verified by maximal customer satisfaction in many European countries.
Contact us

For non-binding offer please contact us on:
Company INOX Processing – Milan Svoboda is following highest standards and norms for effective production and best quality of the products. We follow these authorities: